Which of the Following Molecules Contains a Nonpolar Covalent Bond
Hydrogen sulfide has been consisted of the highly electronegative Sulfur and Hydrogen bond thus have been able to form the polar covalent bond. Predict the shape and bond angle for the compound carbon tetrafluoride CF4.

Covalent Bonds Biology For Majors I
NaF Br2 HCl O2 MgO.
. The VSEPR theory allows us to determine the. Which are overall nonpolar molecules. Share pair of electrons with H to make a coordinate bonds.
O CC14 O CHCI3 O BH3 O H20 O CO2. The Lewis structure for the polyatomic ion NO 2 contains a 2 double bonds and 8 nonbonding electrons. Shape of a molecule.
Thus C H 4 is a nonpolar molecule. When a covalent bond joins atoms of different elements and the bonding electrons are shared unequally the bond is. Thus the bond has been a covalent nonpolar bond.
All the atoms attached to the middle atom are identical. 2 P2O5 covalent compound diphosphorus pentoxide or more usually phosphorus V oxide. Almost all the covalent bonds in lipids are nonpolar causing their solubility in water to be extremely low.
Lipids are hydrophobic substances. The atoms attached to the atom arent all the same. The bond Cl2 is a.
2 KCl Ionic bond between K and Cl. Which of the following has nonpolar bonds. The arrows are equal in length and the arrangement is symmetrical.
Thus nitrogen from NH 3. Is electron rich and H ion do not contain any electron. Which of the following molecule possesses polar and nonpolar covalent bond.
Which of the following molecules contains a nonpolar covalent bond. A nonpolar molecule with polar bonds. You must draw Lewis structures and determine shape before answering 1st attempt Choose one or more.
D d- The fluorine end of the molecule has higher electron. The following molecules contain polar covalent bonds. In covalent compounds some ionic character exists.
H 2 and C l 2 molecule contains Non polar covalent bond. In which of the following molecules is a coordinate covalent bond present. Trigonal planar 120 degrees.
1 1 pts Question 24 Which of the following molecules has the largest dipole moment. The donor atom should have at least one lone pair of electrons and the acceptor atom should have at least one vacant orbital. Chlorine gas has nonpolar bonds.
Coordinate bond is formed between electron rich and electron deficient atoms. Nonpolar covalent bond. The shape of the ammonia molecule NH3 is.
C 2 single bonds and 12 nonbonding electrons. Carbon dioxide contains polar covalent bonds but is not polar overall due to symmetry. PH 3 Covalent bond between P and H.
O 2 Covalent bond between O atoms. State whether the following compounds contain polar covalent bonds non polar covalent bonds or ionic bonds based on their electronegativities. Which are overall nonpolar molecules.
B 2 H 6 Covalent bond between B and H atoms. The bond in carbon tetrachloride has been based on the electron sharing between the electronegative elements. The arrows are of different lengths and the arrangement is asymmetrical or uneven.
A NH4Cl b CCI3 c H2O2 d HCN. Asked Jan 31 2021 in Biology Microbiology by Emilio. Hence only H 2 O 2 molecules have both polar and nonpolar bonds and hence the correct option is C.
The name of the HSO4- ion is sulfite. Which of the following compounds contains a polar covalent bond. Which of the following compounds contains a polar covalent bond.
Polar and Nonpolar Covalent Bonds In many molecular compounds however one atom attracts the bonding electrons more strongly than the other. Also hydrogens are evenly distributed around carbon so all the poles cancel out each other. When a covalent bond is formed between two atoms of the same element the shared electron pair will lie exactly midway between the two atoms iethe electrons are equally shared by the atoms.
Boron trichloride contains polar covalent bonds but is not polar overall due to symmetry. Classify each of the following compounds as ionic covalent or polar covalent. D d-Fluorine attracts electrons H F more strongly than hydrogen.
Mixed IonicCovalent Compound Naming For each of the following questions determine whether the compound is ionic or covalent and name it appropriately. A H b c d O 7. A polar molecule with nonpolar bonds.
H 2 SO 4 Covalent bond between S and O and also between O and. The following molecules contain polar covalent bonds. A nonpolar molecule with nonpolar bonds.
They share all electron pairs. Which of the following compounds contains one or more covalent bonds. B 1 double bond 1 single bond and 12 nonbonding electrons.
No compound is completely covalent or ionic.

Nonpolar Covalent Bond Definition And Examples

Lesson Explainer Polar And Nonpolar Solvents Nagwa

Polar And Nonpolar Covalent Bonds Ppt Download
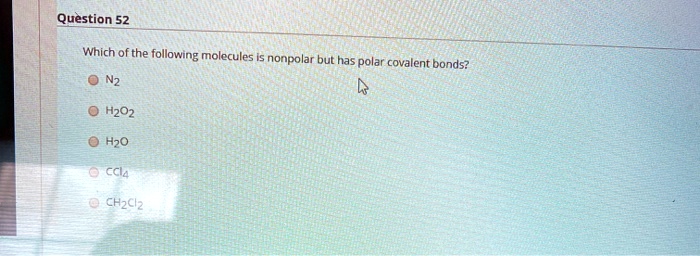
Solved Question 52 Which Of The Following Molecules Nonpolar But Has Polar Covalent Bonds H202 H2o Cci4 Chzclz

Polar And Nonpolar Molecules Youtube

Polar Vs Nonpolar Covalent Bonds Examples What Are Polar Nonpolar Covalent Bonds Video Lesson Transcript Study Com

Polar And Nonpolar Molecules How To Tell If A Molecule Is Polar Or Nonpolar Youtube
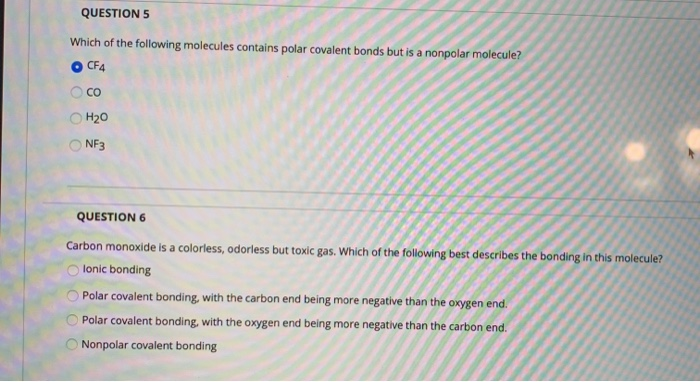
Solved Question 5 Which Of The Following Molecules Contains Chegg Com

A Molecule That Has A Symmetrical Distribution Of Polar Covalent Bonds Will Be Non Polar As A Whole Quora

Pin By ᝰ Fifi On Study Motivation In 2022 Covalent Bonding Hydrogen Bond Surface Tension
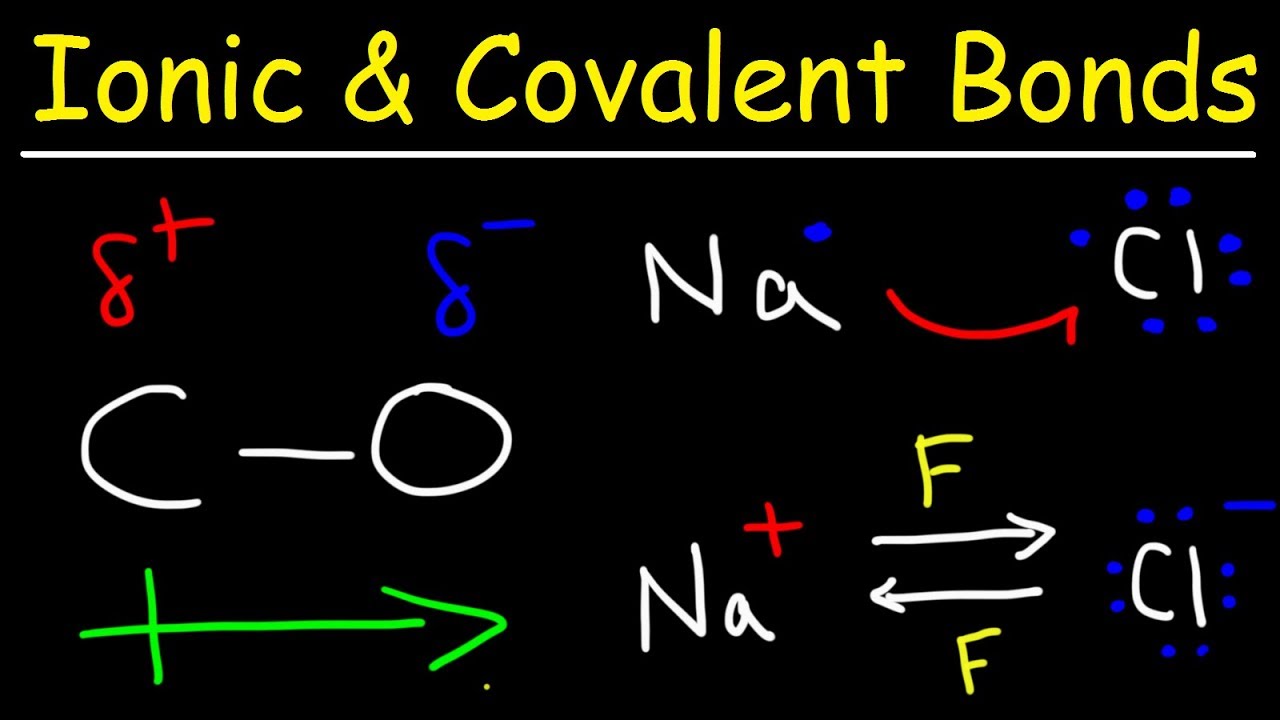
Ionic Bonds Polar Covalent Bonds And Nonpolar Covalent Bonds Youtube

Is Scn Polar Or Nonpolar Covalent Bonding Molecular Geometry Solubility
Solved Which Of The Following Molecules Has The Most Chegg Com

Nonpolar Covalent Bond Definition And Examples

Polar And Nonpolar Covalent Bonds Ppt Download

Polarity Of Molecules Molecules Covalent Bonding Thought Process

Bond Polarity Chemistry For Non Majors

Nonpolar Covalent Bond Covalent Bonding Chemistry Basics Chemistry Lessons
